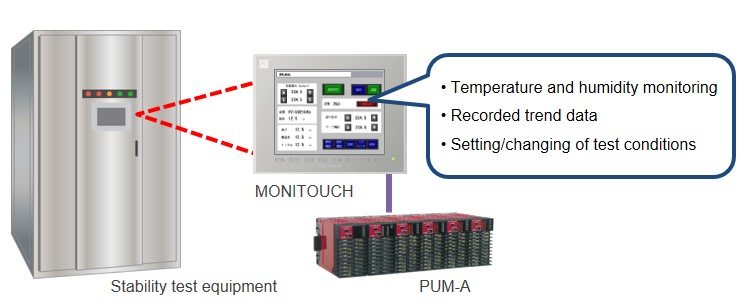
Stability test equipment and stability test chambers are environmental devices that are able to control temperature and humidity. These devices maintain storage space in a stable state are used to test pharmaceuticals under certain environmental conditions for the purpose of detecting minute defects in formulations.
- It is a requirement in the pharmaceutical industry that information on manufactured pharmaceuticals is retained for records.
- Recently due to the enactment of Title 21 CFR Part 11, digitization of these records has been approved and there are various methods that customers are using for record management.
- MONITOUCH can print out documents that are necessary for record management.
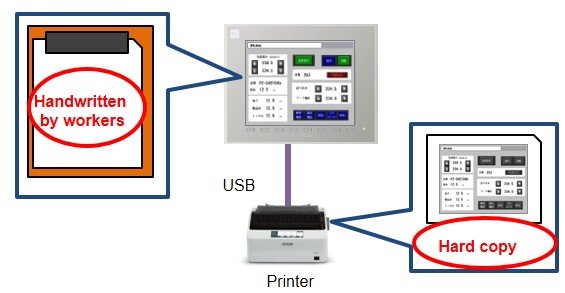
Before
Customer A: Various test data was stored on the touch panel but controlled documents were created by hand.
Customer B: Screens for printing were prepared separately on the touch panel and printed hard copies were used as controlled documentation.
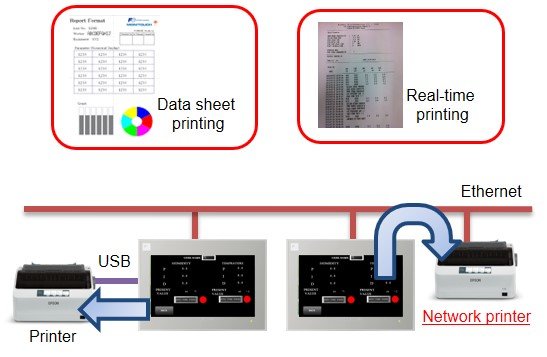
After
MONITOUCH supports real-time printing. Recorded data and records of alarms that occurred during batch processing can be printed on the same page by online (real-time) printing.
MONITOUCH is provided with a data sheet function as a standard feature. Data sheets can be displayed on MONITOUCH and printed using formats designed in advance.